Electrolytes
Electrolytes are a substance that produces an electrically conducting solution when dissolved in a polar solvent, such as water. The dissolved electrolyte separates into cations and anions, which disperse uniformly through the solvent. Electrically, such a solution is neutral. If an electrical potential (voltage) is applied to such a solution, the cations of the solution would be drawn to the electrode that has an abundance of electrons, while the anions would be drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. This includes most soluble salts, acids, and bases. Some gases, such as hydrogen chloride, under conditions of high temperature or low pressure can also function as Electrolytes. Electrolyte solutions can also result from the dissolution of some biological (e.g., DNA, polypeptides) and synthetic polymers (e.g., polystyrene sulfonate), termed polyelectrolytes, which contain charged functional groups. A substance that dissociates into Ions in solution acquires the capacity to conduct electricity. Sodium, potassium, chloride, calcium, and phosphate are examples of electrolytes, informally known as lytes.[1]
Human Body Electrolytes
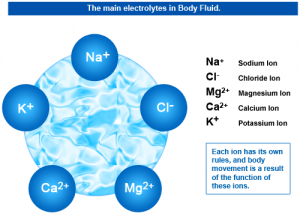
The human body is made up of atoms, Ions and molecules. Ions are atoms with extra electrons or missing electrons. When an atom is missing an electron or two, it has a positive charge. When an atom has an extra electron or two, it has a negative charge. The key players in creating the electrical energy within our body are structures called ions. Ions may exist in solid, liquid, or gaseous environments, although those in liquid are more common. Most ionic compounds fall in the category of chemicals called salts. Ions existing in a liquid state are Electrolytes. An electrolyte is any compound that, in solution, conducts electricity and is decomposed or electrolyzed by the electricity.[2]
Ionic compounds exist as crystals rather than molecules, and are dissolved in the fluids of the human body creating cell salts. The ionic bonds help to create cell salts, which give strength to the tissues of the human body, increasing Cellular Integrity. Each ion has its own properties and rules, which perform specific body movements, which are a result of the function of these Ions or cell salts. Ionic compounds that break down when dissolved in water are called electrolytes. Electrolytes are strongly conductive and have important functions in the body:
- Water management functions in the body, hydration
- Produce nerve impulses, essential for muscle function
- Transport nutrients to the cell
- Maintain blood pH levels
- Support mental functioning through strengthening neural net
- Convert calories into usable energy more efficiently
- Build foundation for the enzyme activity in the body
- Catalyze the process of biological ionization (Ascension)
- Help to control the flow of current, in and out of cells
It is clear that imbalances of any of these Ions or cellular salts in the body, as well as dehydration, inhibit transport across the cell membranes and can lead to dysfunction in the conduction of electrical messages. This dysfunction quickly leads to a general body disturbance and loss of ability to maintain homeostasis. Therefore, staying well hydrated with as much pure water as possible, and potentially, supplementing with Biochemic Cell Salts and Electrolytes, may be very helpful for an ascending human.[3]